-
Generation Bio Announces Demonstration of Highly Selective T Cell Transduction In Vivo with Cell-Targeted LNP Platform
Источник: Nasdaq GlobeNewswire / 26 окт 2023 06:59:00 America/New_York
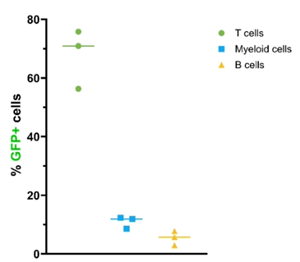
- 70% of circulating and splenic T cells positive for mRNA expression in humanized mice with less than 8% delivery to off-target immune cells
- Stealth properties of cell-targeted lipid nanoparticle (ctLNP) platform confirmed in non-human primates, demonstrating prolonged circulation and less than 0.1% delivery to liver and spleen
- Data provide initial proof of concept for in vivo delivery to T cells and the ability to apply ctLNP platform to extrahepatic tissues and cell types
- Company will present today at the ESGCT 30th Annual Congress and host a webcast R&D deep dive on November 1
CAMBRIDGE, Mass., Oct. 26, 2023 (GLOBE NEWSWIRE) -- Generation Bio Co. (Nasdaq:GBIO), a biotechnology company innovating genetic medicines for people living with rare and prevalent diseases, announced that it has reached two significant achievements through in vivo studies, including results generated as part of its collaboration with Moderna, Inc. First, Generation Bio has shown highly selective T cell targeting in vivo with its cell-targeted LNP (ctLNP) platform in a humanized mouse model. Separately, the company has confirmed the stealth properties of its ctLNP platform in non-human primates (NHPs). Generation Bio will present these data at the European Society for Gene and Cell Therapy (ESGCT) 30th Annual Congress in Brussels, Belgium at 4:40 p.m. CEST today. Slides from the presentation will be made available on Generation Bio’s website following the presentation.
Generation Bio’s ctLNP platform enables delivery of RNA and DNA cargos to difficult to reach tissues and cell types by avoiding off-target clearance by the liver and spleen, and by using conjugated targeting ligands to drive selective, receptor-mediated uptake. The ctLNP platform is the foundation of the immune cell targeting programs in Generation Bio’s collaboration with Moderna, which aims to leverage ctLNPs for in vivo delivery of nucleic acid therapies to select immune cell types, including T cells.
“The data we’ve generated for our ctLNP platform demonstrate a unique ability to target extrahepatic tissues and cell types, giving us confidence as we advance genetic medicines for the treatment of diseases beyond the liver, which is an exciting new frontier for the field and for patients,” said Matt Stanton, Ph.D., chief scientific officer of Generation Bio.
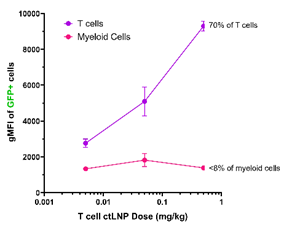
As part of the company’s collaboration with Moderna, Generation Bio has demonstrated that its T cell ctLNPs successfully drive highly selective receptor-mediated uptake and expression of mRNA in humanized mice upon systemic administration, as shown in Figure 1. Furthermore, uptake and expression in T cells was shown to be dose-responsive, without increasing delivery to off-target cell types, as shown in Figure 2.
Figure 1: mRNA expression with T cell ctLNP in humanized mouse model
Figure 2: Dose response with T cell ctLNP in humanized mouse model
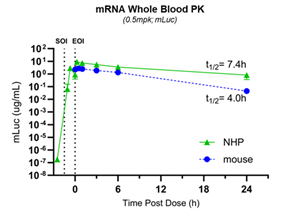
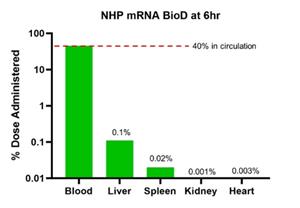
In a separate NHP study, the company confirmed the properties of its stealth LNP, including long circulating half-life and very low off-target delivery to liver and spleen, as shown in Figures 3a and 3b.
Figure 3a: Half-life of stealth LNP in NHPs and mice
Figure 3b: Biodistribution of stealth LNP in NHPs
“The mRNA expression data indicate the power of ctLNPs to de-target the liver and spleen and reach specific extrahepatic cell types by using a targeting ligand,” said Phillip Samayoa, Ph.D., chief strategy officer of Generation Bio. “Furthermore, species translation for the stealth LNP underlying our ctLNP platform is highly encouraging as we continue to move forward with our cell-targeting work. We have made significant advances in bioconjugation and ligand optimization, which we are applying to develop ctLNPs for new targets.”
In addition to today’s presentation at ESGCT, the company will host a webcast-only R&D deep dive and Q&A session on Wednesday, November 1. This presentation will provide further detail on Generation Bio’s ctLNP and iqDNA platforms as well as on its RES manufacturing. Registration information for the webcast R&D deep dive can be found on the Events page of Generation Bio’s investor website.
About Generation Bio
Generation Bio is innovating genetic medicines to provide durable, redosable treatments for people living with rare and prevalent diseases. The company’s non-viral genetic medicine platform incorporates novel immune-quiet DNA construct called iqDNA; a unique cell-targeted lipid nanoparticle delivery system, or ctLNP; and a highly scalable capsid-free manufacturing process that uses proprietary cell-free rapid enzymatic synthesis, or RES, to produce iqDNA. This approach is designed to enable multi-year durability from a single dose, to deliver large genetic payloads, including multiple genes, to specific tissues and cell types, and to allow titration and redosing to adjust or extend expression levels in each patient. RES has the potential to expand Generation Bio’s manufacturing scale to hundreds of millions of doses to support its mission to extend the reach of genetic medicine to more people, living with more diseases, around the world.
For more information, please visit www.generationbio.com.
Forward-Looking Statements
Any statements in this press release about future expectations, plans and prospects for the company, including statements about the company’s strategic plans or objectives, cash resources, technology platform, research and clinical development plans, the terms of the research collaboration between Moderna and Generation Bio to develop novel nucleic acid therapeutics, including the potential to target immune cells with diverse nucleic acid cargos and the liver for gene replacement, the targets to be developed under the collaboration, and the potential benefits and results that may be achieved through other preclinical data and other statements containing the words “believes,” “anticipates,” “plans,” “expects,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: that the anticipated benefits of the collaboration with Moderna may not be achieved on the anticipated timeline, or at all; that data may not support further development of the therapies subject to the collaboration with Moderna due to safety, efficacy, or other reasons; uncertainties inherent in the identification and development of product candidates, including the conduct of research activities, the initiation and completion of preclinical studies and clinical trials and clinical development of the company’s product candidates; uncertainties as to the availability and timing of results from preclinical studies and clinical trials; uncertainties regarding our novel technologies; whether results from earlier preclinical studies will be predictive of the results of later preclinical studies and clinical trials; uncertainties regarding the RES manufacturing process; challenges in the manufacture of genetic medicine products; whether the company’s cash resources are sufficient to fund the company’s operating expenses and capital expenditure requirements for the period anticipated; the impact of the COVID-19 pandemic on the company’s business and operations; as well as the other risks and uncertainties set forth in the “Risk Factors” section of our most recent annual report on Form 10-K and quarterly report on Form 10-Q, which are on file with the Securities and Exchange Commission, and in subsequent filings the company may make with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the company’s views as of the date hereof. The company anticipates that subsequent events and developments will cause the company’s views to change. However, while the company may elect to update these forward-looking statements at some point in the future, the company specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the company’s views as of any date subsequent to the date on which they were made.
Investors and Media Contact
Maren Killackey
Generation Bio
mkillackey@generationbio.com
857-371-4638Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/9dedd290-1c0c-4c1a-9ef3-6bba7dabf2e4
https://www.globenewswire.com/NewsRoom/AttachmentNg/f445317c-469f-49a0-b192-1b06c2814cdf
https://www.globenewswire.com/NewsRoom/AttachmentNg/6f8eb38a-ebb0-4c98-9ef6-0195114b3fcd
https://www.globenewswire.com/NewsRoom/AttachmentNg/51f92b08-cb41-468e-b96f-64be220b77a7
- 70% of circulating and splenic T cells positive for mRNA expression in humanized mice with less than 8% delivery to off-target immune cells